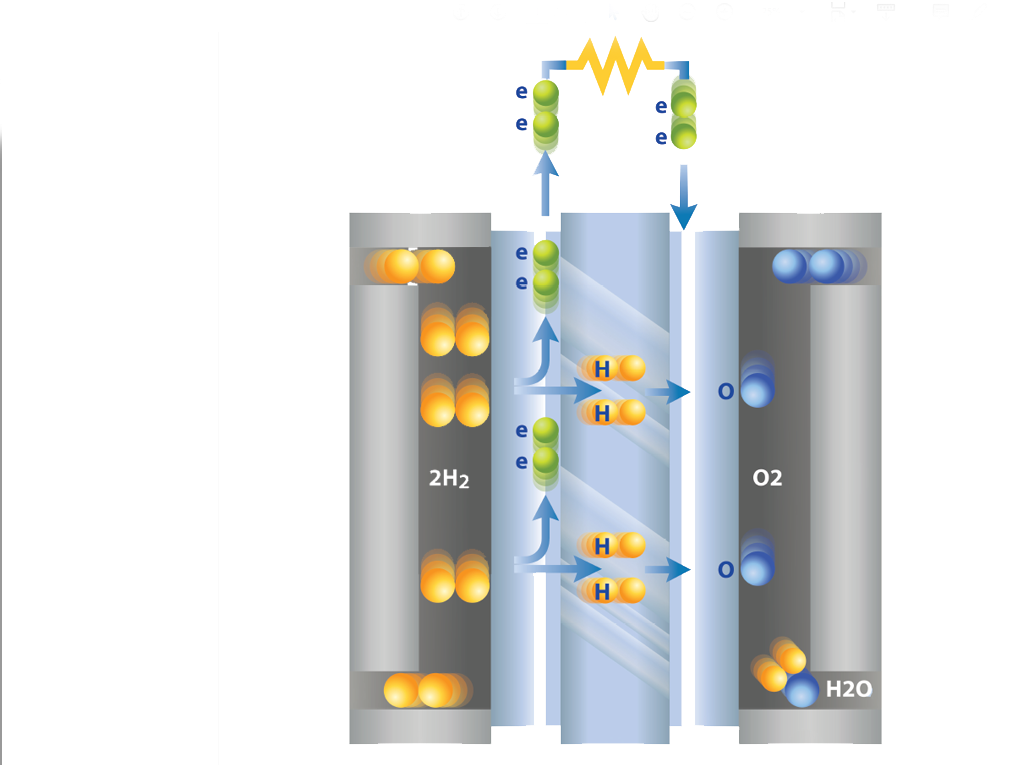
A fuel cell is a device that generates electrical energy from chemical reactions. As such, a Proton Exchange Membrane (PEM) fuel cell generates electricity through a chemical reaction between hydrogen and oxygen. The fuel cell requires a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction. Through this reaction fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. This electrochemical process means that the fuel cells operates without pollution with the only by-products being water and heat. This makes fuel cells an ideal part of the transmission to a zero-emission society.
The fuel cell technology has a long history: it was discovered by Sir William Grove in 1839. Since then there has been developed a range of different types of fuel cells. These include Solid Oxide, Alkaline and Proton Exchange Membrane (PEM) fuel cells. The fuel cells developed by Ballard Power Systems and integrated in all its modules are entirely based on the PEM technology. The PEM fuel cell was first used by NASA in the 1960s as part of the Gemini space program. NASA´s interest in fuel cells as well as the energy crisis in 1973 pushed the further development of fuel cells.
Today this technology is used to power a large variety of motive applications including cars, trucks, forklifts, trains and ships, as well as stationary back up power systems.
The catalytic membrane is the most important component of the PEM fuel cell, that´s why Ballard´s research and development efforts are constantly concentrated on developing efficient, durable and cheap material to reduce the overall cost of the fuel cell.
The advantages of the PEM fuel cell compared to the other fuel cells includes:
- The low operating temperature, which simplifies materials issue, provides for quick start up and increases safety
- The low operating pressure, and a consequent increased safety of the system
- The high voltage, current and power density
- The high tolerance of carbon dioxide. As a result, PEM fuel cells can use un-scrubbed air as oxidant, and reformate as fuel
- The use of a non-corrosive electrolyte. Pure water operation minimizes corrosion problems and improves safety
Ballard fuel cells are well proven having been fitted to over 70 buses and provided over 14 million kilometres of reliable service.